|
Structure and Virology |
Human respiratory syncytial virus (RSV), which causes illness in people’s lungs and breathing passages, is a highly contagious virus worldwide, and typically circulates during the late autumn and winter months. It is an enveloped, non-segmented negative-strand linear RNA genome virus belonging to the family Paramyxoviridae, subfamily Pneumovirinae, genus Orthopneumovirus. In addition to RSV, the Paramyxoviridae family includes other important human and animal pathogens, such as the Nipah, measles virus, canine distemper virus, and Newcastle virus as well. The RSV virion particle is pleomorphic with a diameter of 150–300 nm and is composed of a helical nucleocapsid packaged in a lipid envelope derived from the host cell membrane. Three transmembrane glycoproteins: the fusion protein (F), the glycoprotein (G), and the small hydrophobic (SH) protein are located on the virus lipid envelope (Fig 1).
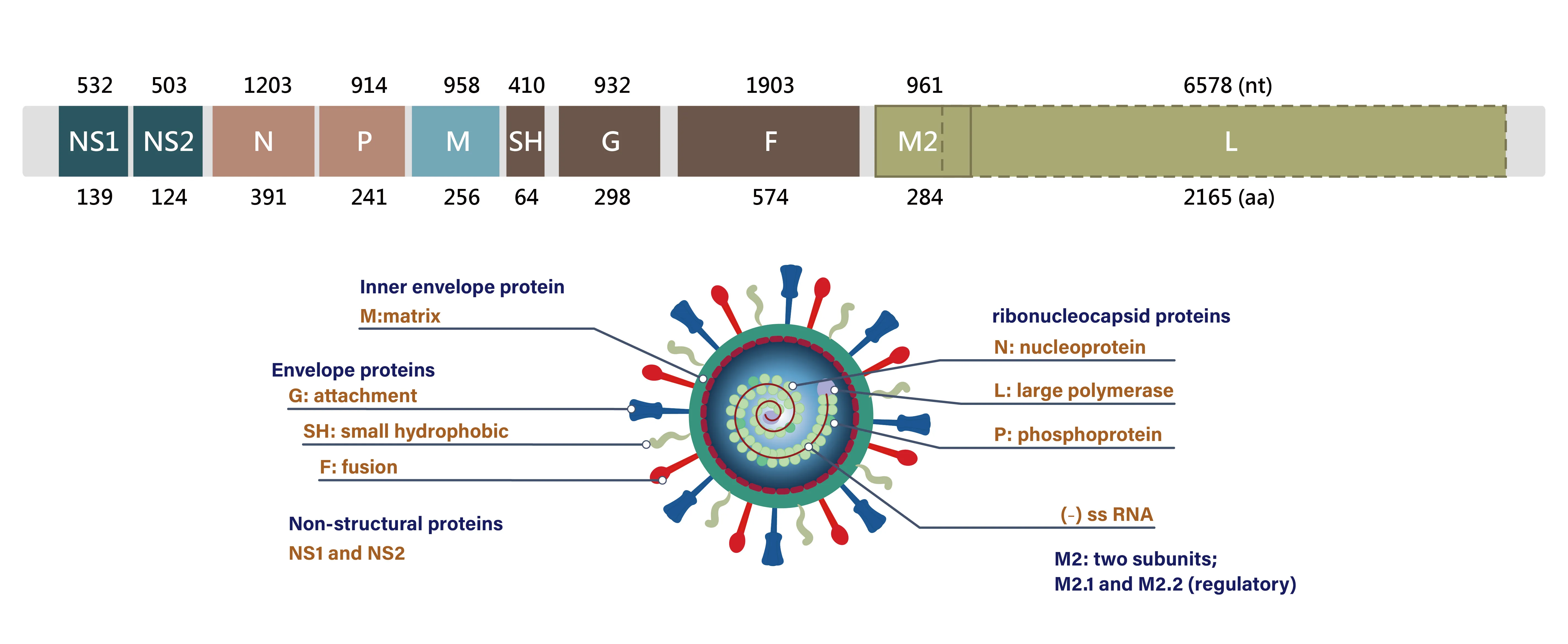
Fig 1. RSV Genome Structure
The entire genome of RSV is composed of approximately 15,000 nucleotides and contains 10 genes encoding for 11 proteins, two of which are nonstructural (NS1 and NS2). There are two major antigenic groups (A and B) that have been identified according to cross-reactivity patterns of the F and G integral proteins to monoclonal antibodies (mAbs). Differential contributions in viral infection have been studied, and it was found that the G protein facilitates viral attachment via interaction with glycosaminoglycans on cilium cell surfaces, while the F protein mediates fusion between the virion, the host cell, and the adjacent cell membranes. The formation of multinucleated giant cells called syncytia hallmarks a characteristic cytopathic effect of RSV; hence, viral RNA can spread without forming complete virion particles, and that is how it has gained its name.
|
Major symptoms and prophylaxis |
Clinical symptoms can range from mild cold-like sickness to severe respiratory failure. Recurrent infections of RSV are common during one’s lifetime. Most people show nearly universal rhinorrhea, cough, and low-grade fever after an incubation period of 2 to 8 days, and they recover in two weeks, but certain vulnerable groups of patients are at risk of developing a serious illness because viruses replicate in the bronchi and bronchioles.
Since its discovery in 1956, RSV infection has become a leading cause of emergency department visits and hospitalizations in infants and children, especially during their first 6 months of life or before reaching the age of two.
Substantial mortality alongside morbidity among the elderly with debilitating conditions and immunocompromised individuals are stated owing to generalized and intense inflammatory damage in the lungs. Besides acute manifestations of bronchiolitis and pneumonia, RSV may exacerbate chronic medical conditions e.g., asthma, chronic obstructive pulmonary disease, and congestive heart failure.
Both RSV serogroups A and B cocirculate in the community and studies conducted in diverse regions of the world reveal consistent shifts or a balance in RSV group dominance. Recurrent infections indicate that different strains share only partial cross-immunity.
Sequence variation of the RSV-G gene and amino-acid or glycan-linked antigenic sites respectively produces multiple RSV genotypes and presumably weakens preexisting host immune responses, which in turn enhances virus pathogenicity. In contrast, the trimeric RSV-F protein is relatively conserved among isolated strains from both A and B subgroups, with amino acid sequence identities of 90% or higher.
Although natural RSV infection appears to confer partial and nondurable immunity, neutralizing antibodies against the RSV-F protein can prevent severe disease by passive prophylaxis with the F-directed mAbs like palivizumab and nirsevimab.
Therefore, the RSV-F protein is likewise a promising target for vaccine development, but it should undergo conformational changes and be antigenically distinct between the prefusion and postfusion states. Isolated antibodies specially binding to epitopes on RSV-F protein that are only exposed at the metastable prefusion conformation have been reported to display satisfactory neutralizing activity, outperforming previous ones.
|
Diagnosis |
Accurate and prompt diagnosis not only can precondition therapeutic strategies so as to protect the lives of humans and reduce the burden on the healthcare system, but also contribute in having a timely response towards future emerging respiratory virus epidemics. Recent advances in point-of-care tests (POCTs) including nucleic acid amplification and dipstick-based immunoassays improved shortcomings in terms of turnaround time and performance.
The reverse transcription-PCR (RT-PCR) technique is the most widely utilized tool for diagnosing and genotyping RSV infections. In the past decade, multiplex and quantitative real-time RT-PCR (qRT-PCR) assays have been developed to detect nucleic acids extracted from a swab sample suspected of various respiratory viruses, thus clarifying suspicion cases with overlapping symptoms or those that are asymptomatic. Lateral flow immunoassay (LFIA), also known as rapid detection test (RDT), allows for the rapid, qualitative detection of RSV antigen (viral F protein) directly from nasopharyngeal swabs, aspirate, or wash specimens for symptomatic patients. The claimed sensitivity and specificity are normally above 90% but vary considerably depending on the manufacturer. By attaining some certifications of manufacturing practices, a manufacturer can provide proof of quality for their products. Modifications of LFIA may be through adopting chromogenic nanoparticle catalysts or reselecting capturing antibodies.
