-
Species of Origin
Human
Expression System
Escherichia coli
-
Affinity Tag
His Tag (C-term)
Storage Buffer
Lyophilized from a 0.2 µm filtered solution of PBS, pH 8.0.
-
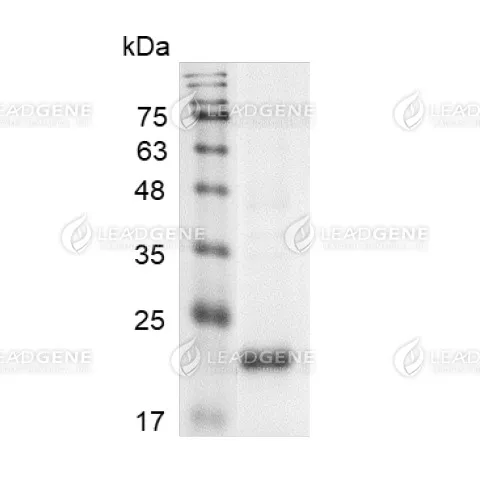
Purity
>98% as determined by SDS-PAGE analysis.
Molecular weight
The protein has a calculated MW of 21.8 kDa.
The protein migrates as 22 kDa under reducing condition (SDS-PAGE analysis). -
Activity
Measure by its ability to induce proliferation in TF-1 cells The ED₅₀ for this effect is < 0.5 ng/mL. The specific activity of recombinant human IL-6 is approximately > 1 x 10⁸ IU/mg, which is calibrated against the human IL-6 WHO International Standard (NIBSC code: 89/548).
Endotoxin Level
<0.05 EU per 1 µg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Lyophilized
-
Specifications
-
Background
-
Background
Interleukin-6 (IL-6) is a pleiotropic, 22-28 kDa cytokine which plays fundamental role in the acute phase response, inflammation, bone metabolism, lymphocyte differentiation and cancer progression. Deregulation of IL-6 production was also found in several diseases, including rheumatoid arthritis, Alzheimer’s disease, autoimmune deficiency disease and different types of cancer.
Synonyms
Interleukin-6, B-cell stimulatory factor 2 , BSF-2, CTL differentiation factor , CDF, Hybridoma growth factor, Interferon beta-2 , IFN-beta-2
-
Uniprot ID
P05231
Sequence Note
Val30-Met212
-
-
Instruction
-
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
-
Stability & Storage
This product is stable after storage at:
- -20°C for 12 months in lyophilized state from date of receipt.
- -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Manufacturing Specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/1 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1
Disclaimer:For Research Use or Further Manufacturing Only.
