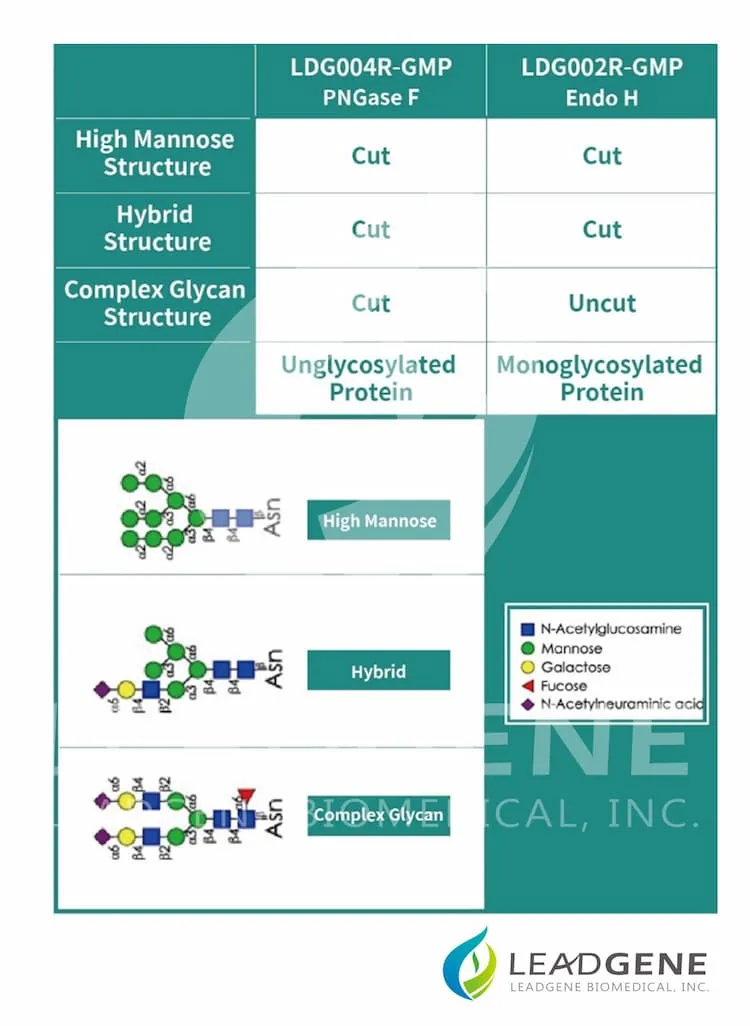
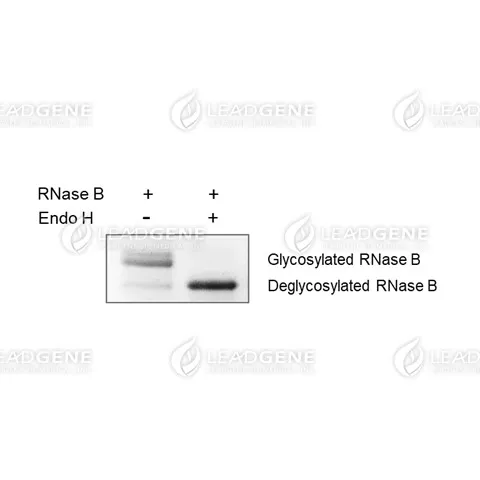
Product Note
- After thawing, the buffers may crystallize, which is a normal occurrence. Warm crystallized buffer until the salt crystals return to solution. Ensure that your components return to RT before use in the assay.
- Please fine-tune the input sample volume to find the optimal condition for your assay.
- Once optimize for the cleavage condition, the cleavage reactions can be scaled up to cleave a large amount of the target fusion protein.
Components
LeadGMP® Endoglycosidase H (Endo H) (500 U/μL)
Quantity
1 vial (50,000 U)
10× Glycoprotein Denaturing Buffer
Quantity
1 vial (1 mL)
10× Reaction buffer
Quantity
1 vial (1 mL)