TAINAN, Taiwan, August 30, 2022 (Newswire.com) - Leadgene Biomedical, Inc., an ISO-certificated contract development and manufacturing organization (CDMO) company that provides integrated service for in-vitro diagnostics (IVD) products from the development to manufacturing process, has announced the launch of its LEADSPHERE™ Lyophilization Technology to aid in the dispense, storage, and transport of reagents for use in biomolecule analysis tools.
"Leadgene has established a reputation for providing a comprehensive solution for biotech companies in the area of high-performance assay kits development," said Dr. Yung-Chun Chuang, Chairman of the Board and Chief Technology Officer of Leadgene, "LEADSPHERE™ Lyophilization Technology enables assay reagents to be stable in non-liquid form at room temperature, and allows for the assembling of the entire kit in a large-scale-production manner through a 'pick and place' automation system."
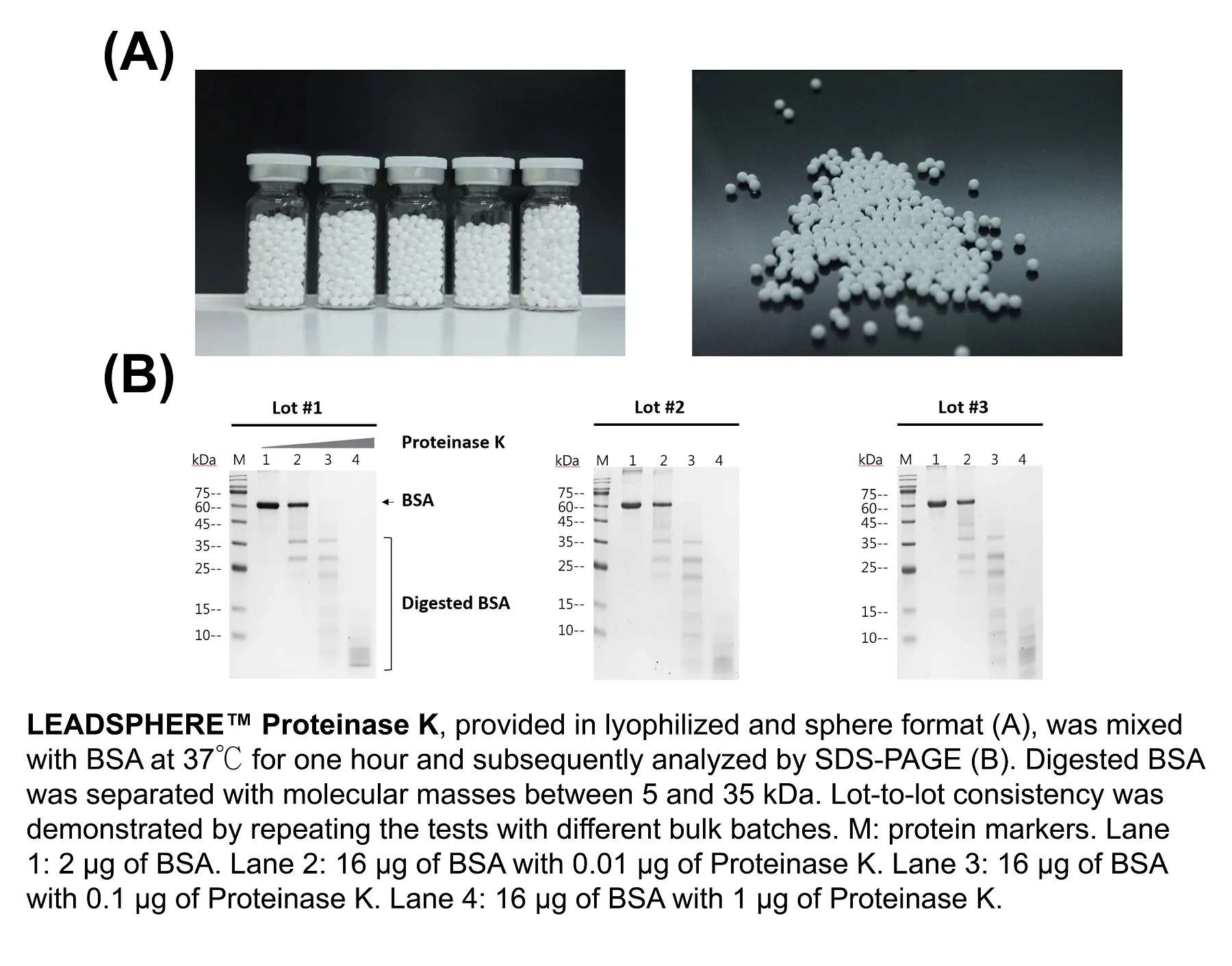
Numerous advantages of freeze-drying reagents in sphere format have been documented, with the most significant one being the ability to sustain the ingredients' activities at room temperature. LEADSPHERE™ Lyophilization Technology is an automated dispensing system composed of all the necessary parts to freeze dry materials in low volume (10~20 microliter). The final product is a stable lyophilized sphere with a consistent diameter of ~3 mm, and therefore, makes it suitable for all sizes of microplates and microtubes. A successful example that utilizes LEADSPHERE™ Lyophilization Technology is the LEADSPHERE™ Proteinase K, an endopeptidase used for nucleic acids sample preparation by digesting proteins and inactivating ribonuclease. The features of this product include:
- Facilitation of long-term logistics at room temperature.
- Ease in aliquoting and handling during the manufacturing process.
- Simplifying point-of-care protocol for end-users.
LEADSPHERE™ Proteinase K has been thoroughly used in applications to identify viruses, including SARS-CoV-2.
About Leadgene Biomedical Inc.
Leadgene Biomedical, Inc. was founded in 2013 and led by a group of young scientists. It established its refined facilities in Taiwan to provide quality reagents and services for the biomedical industry through creative and scientific approaches with standards outlined in quality management systems ISO 13485:2016, and QMS/GMP (Quality management system/ Good manufacturing practices for medicinal products). Along with its CDMO service, Leadgene's catalog products include but are not limited to cytokines, chemokines, growth factors, enzymes, antibodies, and reagents for toolkits.
Contact:
Leadgene Biomedical, Inc.
+886-6-2536677
[email protected]
Press Release Service by Newswire.com
Original Source: Leadgene Utilizes LEADSPHERE™ Lyophilization Technology to Make Reagents Stable for Long-Term Logistics
