-
Species of Origin
Human
Expression system
Escherichia coli
-
Affinity Tag
His Tag (C-term)
Buffer
Lyophilized from a 0.2 µm filtered solution of PBS, pH 8.0.
-
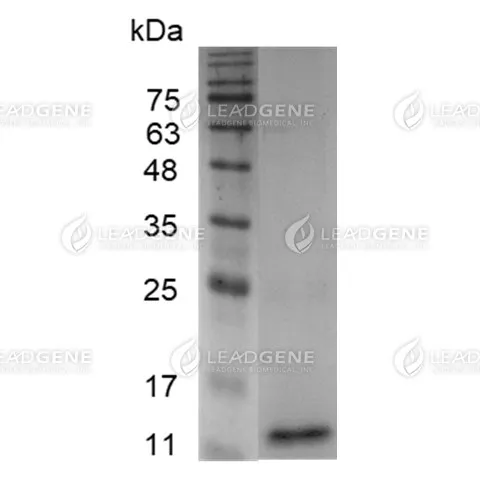
Purity
>98% as determined by SDS-PAGE analysis.
Molecular weight
The protein has a calculated MW of 13.7 kDa.
The protein migrates as 13 kDa under reducing condition (SDS-PAGE analysis). -
Activity
Measure by its ability to inhibit the IL-4 dependent proliferation in TF-1 cells. The ED₅₀ for this effect is <0.1 ng/mL. The specific activity of recombinant human TGF beat 1 is approximately > 1 x 10⁷ IU/mg, which is calibrated against the human TGF Beta 1 Non WHO Reference Material (NIBSC code: 89/514).
Endotoxin level
<0.05 EU per 1 µg of the protein by the LAL method.
-
Mycoplasma
Not detected
Form
Lyophilized
-
Specifications
-
Background
-
Background
The transforming growth factor beta (TGF beta) family of cytokines are ubiquitous, multifunctional, and essential to survival. They play the central roles in growth, development, inflammation, repair, and host immunity. The mammalian TGF beta isoforms (TGF beta 1, beta 2 and beta 3) are secreted as potential precursors and possess a variety of cell surface receptors and produces at least two mediate signal transductions.
Synonyms
Transforming growth factor beta-1 proprotein
-
Uniprot ID
P01137
Sequence Note
Ala279-Ser390
-
-
Instruction
-
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved. In some experiments, it recommends to add 10 mM HCl when reconstitute lyophilized protein.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at -20°C or lower for long term storage.
-
Stability & Storage
This product is stable after storage at:
- -20°C for 12 months in lyophilized state from date of receipt.
- -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Manufacturing specifications
LeadGMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP certified facility. The processes include:
- Animal-free reagent and laboratory
- Manufactured and tested under GMP guideline
- Testing and traceability of raw material
- Records of the maintenance and equipment calibration
- Personnel training records
- Batch-to-batch consistency
- Documentation of QA control and process changes
- Manufactured and tested under an ISO 13485:2016 certified quality management system
- Stability monitor of product shelf-life
-
-
Image
1/1 -
Review

Help others learn more about this product. Use the link below to share your experience.
-
Publication

There are currently no publications. Use the link below to let us know.
-
Datasheet & Documents
1/1
Disclaimer:For Research Use or Further Manufacturing Only.