Cancer is one of the leading causes of death globally, and is predicted to account for an estimated 11.4 million of all deaths annually by 2025.Effective cancer therapy was and still is a major challenge for modern medical research. During the past few decades, numerous efforts have been devoted to the field of immunotherapy to improve the efficacy and safety of conventional therapies, such as radiation and chemotherapy.
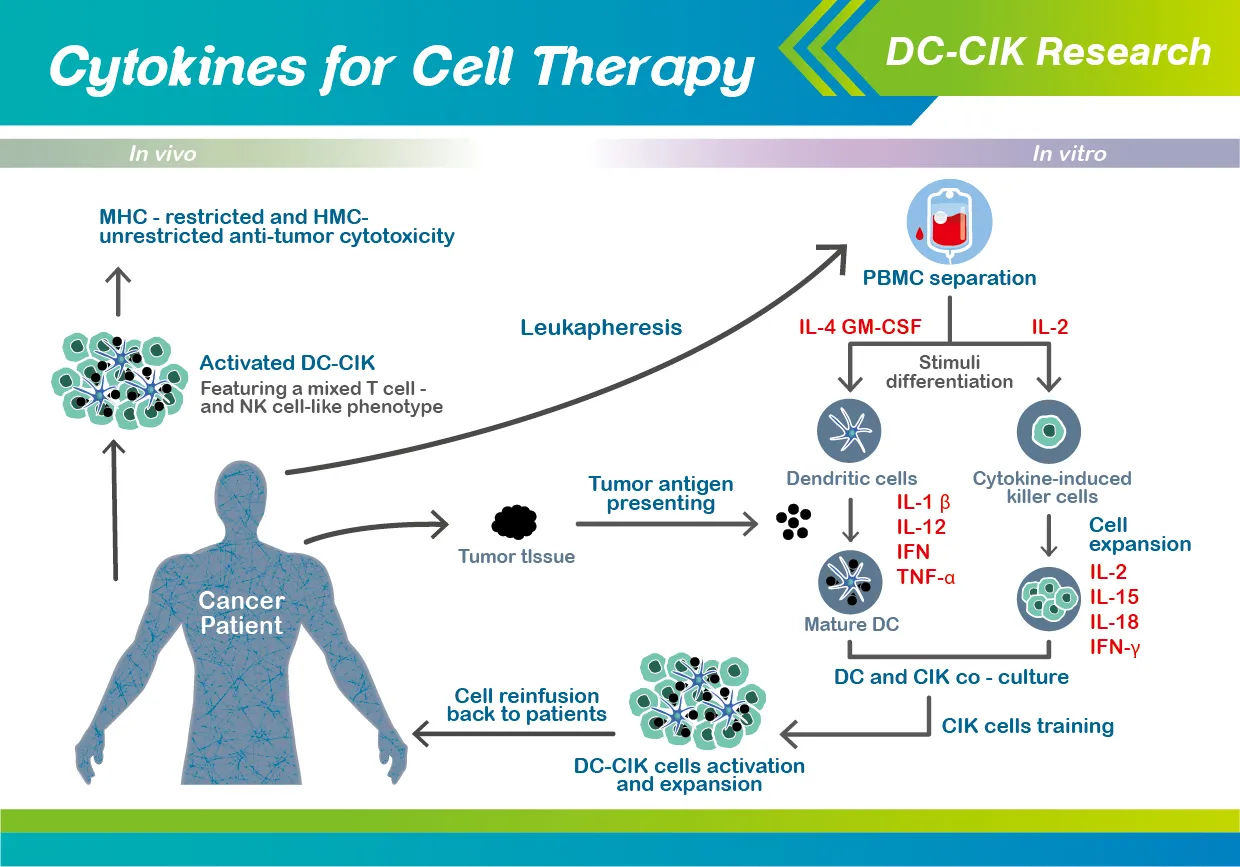
A promising approach to accommodate the need for enhanced cancer treatment is the DC-CIK (dendritic cells and cytokine-induced killer cells) therapy, which is a mixture of in vitro generated cell populations that is first isolated from the peripheral blood of individual patients, and then eventually re-infused back into the same patients in the end.
In addition, Wang et al. extensively reviewed the most recent literatures and clinical trials concerning DC-CIK treatment against solid tumors, which has been present for an extended period of time in various tumor types (including glioblastoma, lung cancer, breast cancer, gastric cancer, pancreatic cancer, hepatocellular carcinoma, and renal cell carcinoma).
|
CT: chemotherapy; MST: median survival time; OS: overall survival time; ORR: objective response rate; RFS: recurrence free survival; Table modified from DC-CIK as a widely applicable cancer immunotherapy.
These recent literature and clinical trials reported that the group of cancer patients who had received the DC-CIK treatment combined with chemotherapy have a significantly longer overall survival rate than the group that had only received the treatment of chemotherapy alone. In another study, an encouraging finding reported that patients who bear pulmonary and mediastinal metastasis of nasopharyngeal carcinoma experienced positive clinical responses following their chemoradiotherapy combined with DC-CIK therapy.
During the follow-up time of 13 years, tumor recurrence or metastasis was not detected. In conclusion, these findings promisingly shed light on the development of cancer therapy, which may provide a higher probability for overcoming cancer mortality. Currently, there are 171 registered studies on adoptive cell therapy (ACT)-treated tumors, and 38 of which are DC-CIK-based regimens. Thus, further investigating various methods for optimal therapy will be a vital task of modern medical research in the time to come. The therapeutic value of DC-CIK therapy is well-anticipated.
References:
- World health organization
- Shuo Wang, et al., DC-CIK as a widely applicable cancer immunotherapy. Expert Opin. Biol. Ther. 2020.
- Gan, et al., Case Report: Chemotherapy and Radiotherapy Combined With DC-CIK for Pulmonary and Mediastinal Metastases From Nasopharyngeal Carcinoma. Oncol. 2022.
- Waldman, et al., A guide to cancer immunotherapy: from T cell basic science to clinical practice. Rev. Immunol. 2020.
