Leadgene Biomedical has been dedicated to protein and antibody development for years. We provide high-quality and highly stable antibodies and recombinant proteins with an ISO13485 certified manufacturing system. We have great innovative energy in research, and we are experienced in platform development. In the COVID-19 research, we have an outstanding ability to help our clients to develop lateral flow platforms.
Leadgene Biomedical has expertise in antigen and antibody development and has obtained several patents worldwide and CE-IVD certification. We manufacture a full panel of premium COVID-19 related products. Leadgene Biomedical is the pioneer in antibody and protein development, and we are your best choice of IVD raw materials.
Product features:
High specificity
High sensitivity
High stability
Comprehensive products
The ISO13485 & GMP certified plant
Rigorous quality control
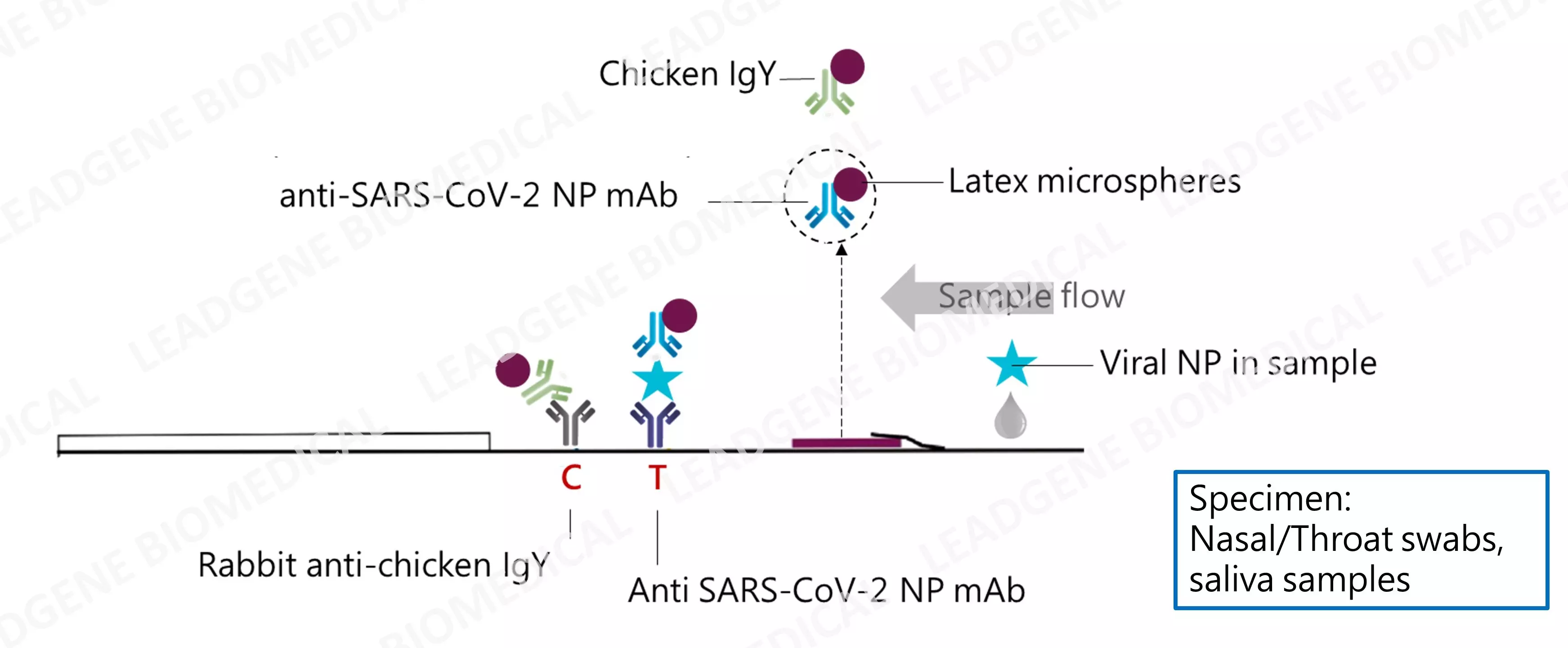
|
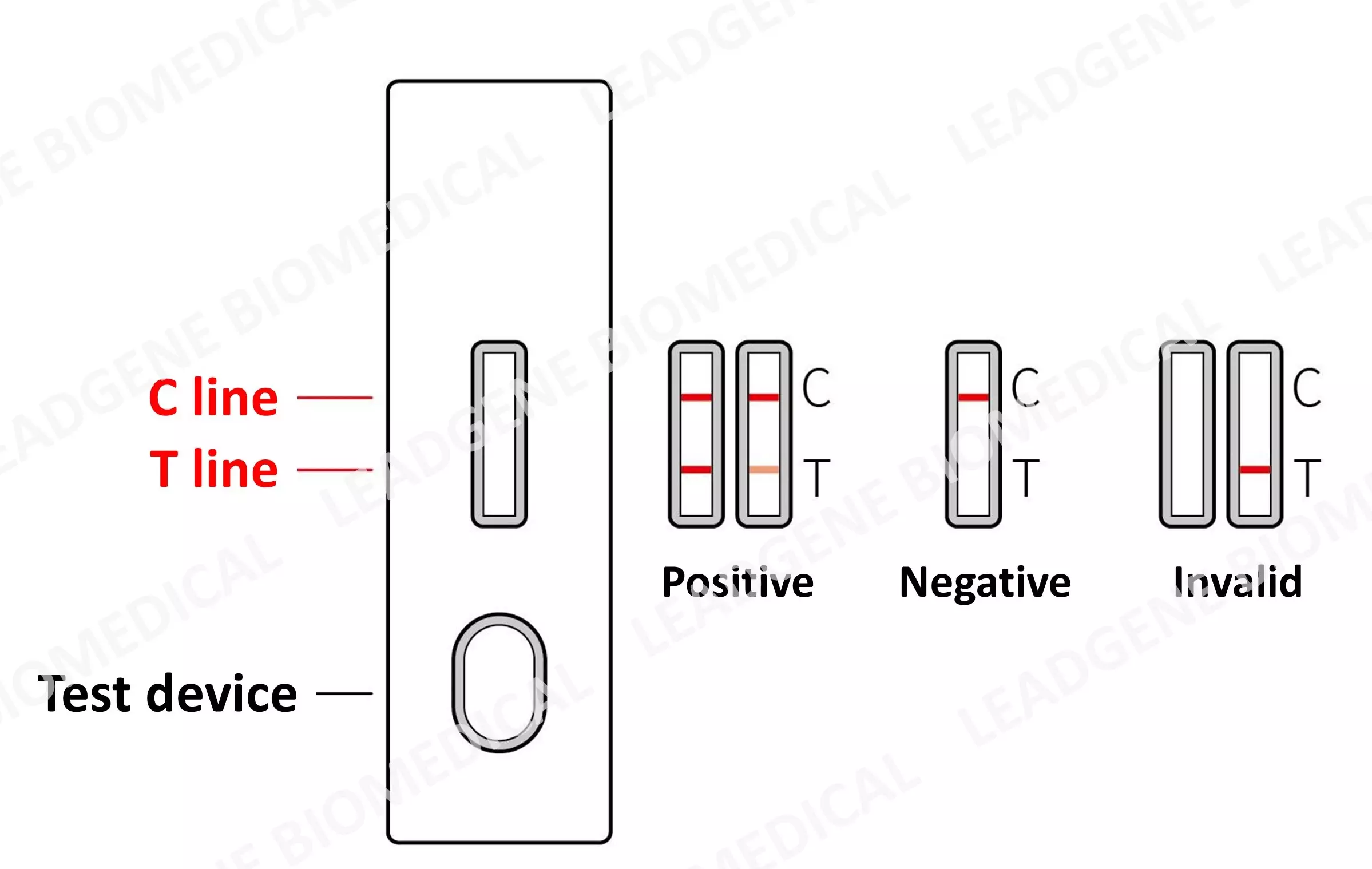
|
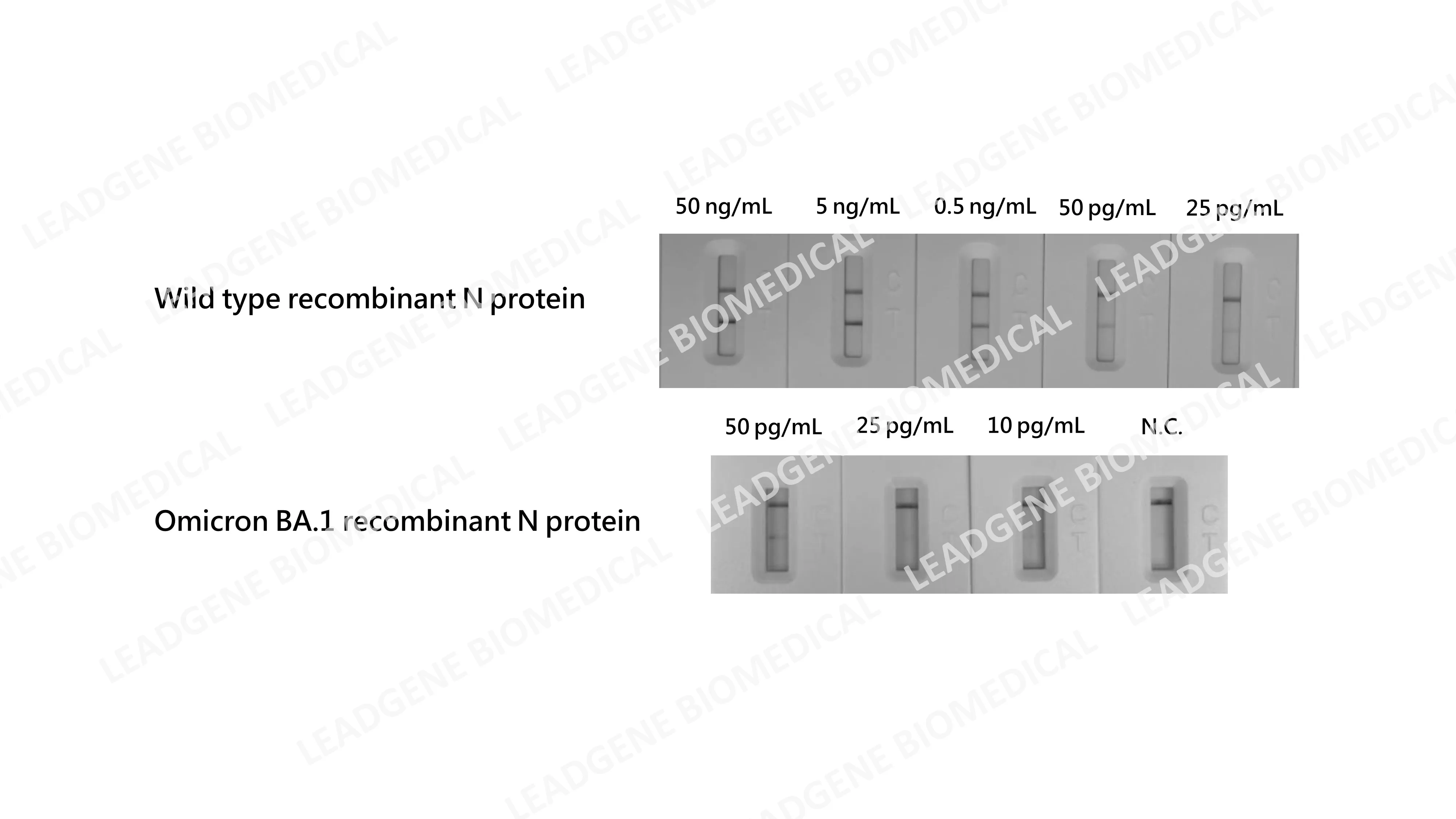
High sensitivity Leadgene develops the highly sensitive SARS-CoV-2 N protein, and the detection limit is as low as 25pg/mL; the detection limit of Omicron N protein achieves as low as 10 pg/mL. |
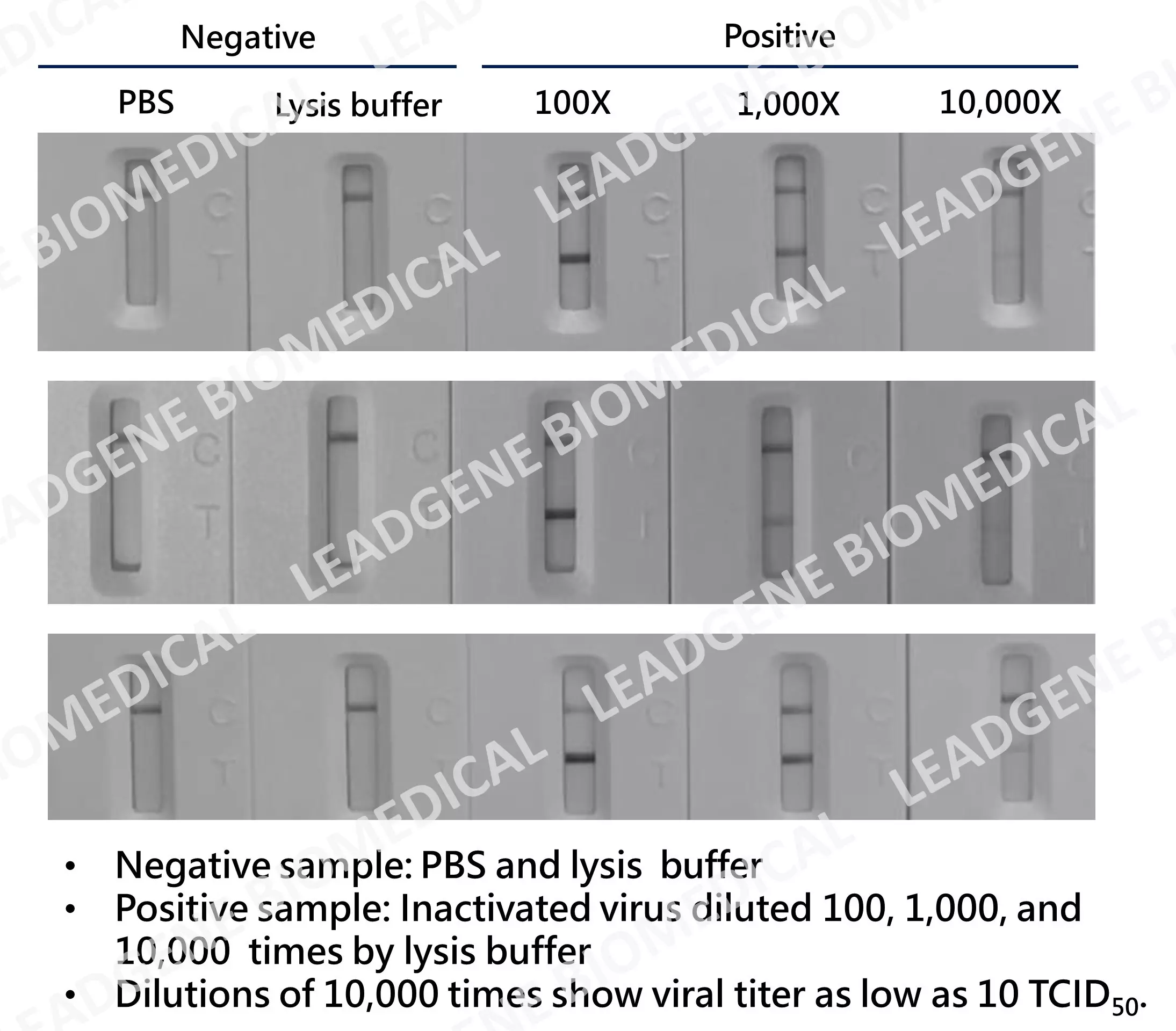
High stability Leadgene develops highly stable anti-SARS-CoV-2 antibodies, which still can recognize inactivated viruses (diluted as low as 10 TCID50) after incubating at 37℃ for 7 days or 14 days. |
