 |
|
||
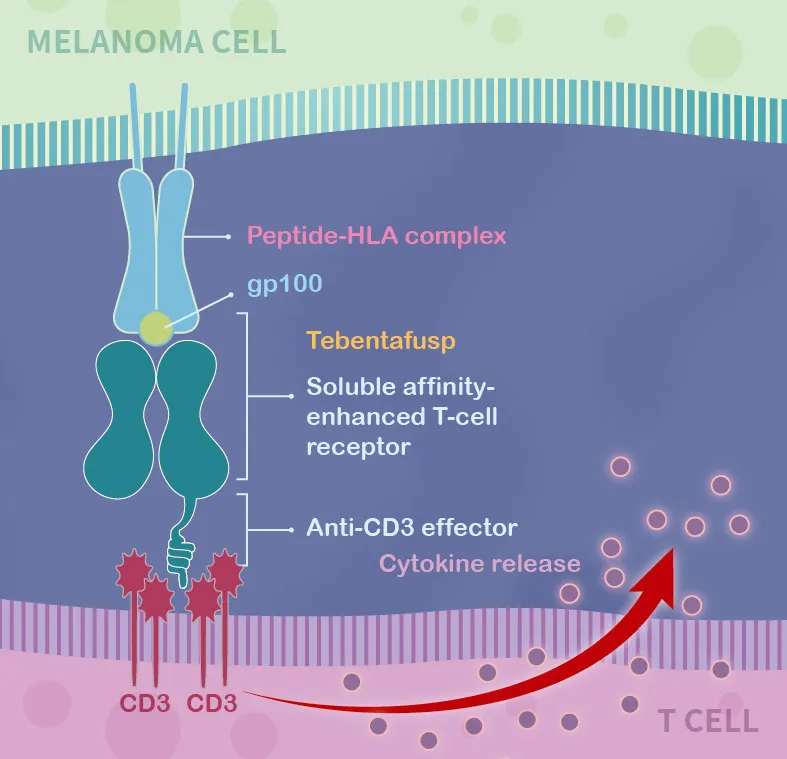
Kimmtrak is a novel bi-specific protein consisting of a soluble affinity-enhanced T cell receptor and an anti-CD3 effector. The soluble affinity-enhanced T cell receptor part can specifically target gp100, a lineage antigen expressed in melanoma; another part of the anti-CD3 effector can recruit and activate T cells to kill tumor cells.
Phase three of using Kimmtrak in the clinical trial was published in the New England Journal of Medicine. Data showed that there was a 73% overall survival rate (OS) for patients that had received the Kimmtrak treatment compared to the 59% OS for patients who received other kinds of drugs. Kimmtrak demonstrated a significant increase in the median of OS.
CAR-T cell therapy has advantages in the treatment of hematological tumors; nonetheless, this treatment is limited to solid tumors. On the other hand, TCR-based therapy is more effective in treating solid tumors; therefore, it is prospectively being studied and developed, and it may potentially become a next-generation strategy for cancer treatment.
References:
- Global Bio & Investment: https://news.gbimonthly.com/tw/article/show.php?num=46074
- GeneOnline: https://geneonline.news/first-tcr-fda-approval/
- Immunocore News Release: https://ir.immunocore.com/news-releases/news-release-details/immunocore-announces-fda-approval-kimmtrakr-tebentafusp-tebn
- Paul Nathan, et al., Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. NEJM. 2021: https://www.nejm.org/doi/full/10.1056/NEJMoa2103485
